The most important property of any emulsion system is its stability. Several different mechanisms exist by which an unstable emulsified state will seek equilibrium.

Why do emulsions destabilise in storage?
1. What is the difference between emulsifiers and emulsion stabilisers?
There are two types of additives that help to stabilise emulsions:
- emulsifiers reduce surface tension, thus lowering the energy required to form droplets.
- emulsion stabilisers prevent the formed droplets from coalescing.
2. Under which conditions are emulsifiers not efficient for stabilising emulsions?
The electrostatic contribution to emulsion stability is strongly reduced in the case of aqueous solutions with high electrolyte contents, especially when multi-valent cations like hard or seawater are present.
Under these conditions, destabilisation of surfactant stabilised emulsions can occur faster and more frequently.
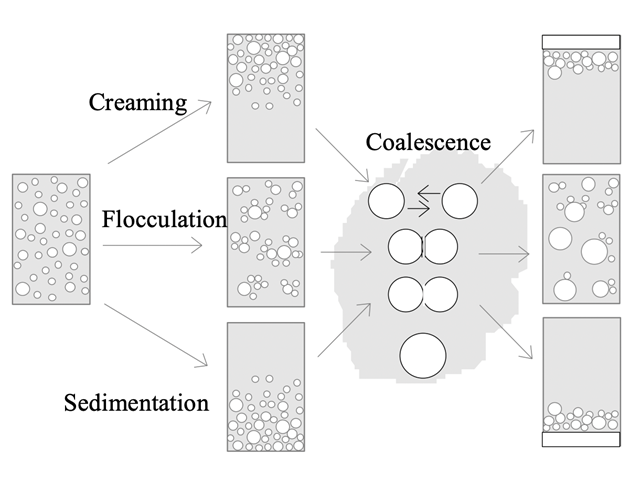
Destabilising process types of emulsions leading to phase separation.
3. How do emulsion stabilisers function?
Several different mechanisms exist for stabilising an emulsion over time without reducing surface tension:
- Introduction of an electrokinetic charge at the o/w interface
- Formation of a semi-rigid film at the interface
- Steric interactions
4. How do biopolymers stabilise emulsions?
Biopolymer molecules, in general, do not possess the amphiphilic structure of conventional surfactants. Even so, as they possess pronounced hydrophobic properties, they will adsorb at the oil/water interface, building up a condensed interfacial film. The viscoelastic (mechanical) properties of this adsorbed layer provide good stability against coalescence.
Our lignin-based products stabilise the emulsion through three combined mechanisms:
- Introduction of an electrokinetic charge at the o/w interface
- Formation of a semi-rigid film at the interface
- Steric interactions
5. What are the advantages of biopolymer-based emulsion stabilisers over emulsifiers?
Emulsions stabilised with biopolymers (and not only with emulsifiers) are more stable over widely varying salinities, temperatures and mechanical actions. The robust nature of the stabilisation mechanism also allows emulsions to be formed with high volume fractions (>70%) of oil droplets.



