The saying, "Oil and water don’t mix" is not completely accurate as oil and water can be mixed as an emulsion.

What is the effect of MFC on emulsion stability
In industries such as the cosmetics, pharma, paints, coatings, household products professionals mix different oils and water to create the desired performance of a product. They overcome the hurdle of "mixing" oil and water by use of emulsifiers, surfactants and stabilisers. So how can MFC contribute?
Here, we will show you an example of why MFC can be considered a stabiliser of emulsions.
What is an Emulsion
An emulsion is a mixture of two or more immiscible liquids. One liquid is dispersed as droplets, the dispersed phase. and the other liquid is the continuous phase. The most common emulsions are either Oil-in-Water (O/W) or Water-in-Oil (W/O) emulsions. More sophisticated systems such as Water-in-Oil-in-Water (W/O/W) and others also exist.
The stability of such systems is ensured by use of emulsifiers, surfactants or stabilisers. These all could be used in a formulation, in some combination or individually depending on the ingredients and conditions that the emulsion will face.
When no stabiliser, emulsifier or surfactant is used, or when an inadequate type is used, an emulsion undergoes creaming, sedimentation, flocculation, coalescence or even phase inversion. In these cases, the emulsion is considered unstable since the dispersed phase is no longer homogeneously dispersed/distributed throughout the continuous phase. This changes the nature of the formulation and very often affects the product’s appearance as well as its performance.
It is, therefore important to avoid such a problem. One way of doing so is through the use of MFC.
MFC as an emulsion stabiliser
Microfibrillated cellulose (MFC) in its native form, is a three dimensional network of cellulose fibrils that can be suspended in water, in a water based formulation or in a polar solvent based formulation. When correctly incorporated into the formulation, MFC results in extremely shear-thinning formulations with very high viscosity at rest. MFC is a high yield stress material and thereby, a very good stabiliser of particles, pigments and droplets of a suspended liquid. All these performances and characteristics are due to the physical network of fibrils.
Due to its very high yield stress provided by the fibril network, MFC can be used as a stabiliser of emulsions. While an emulsifier’s main action is reducing the interfacial tension between the immiscible liquids, the MFC as a stabiliser would form a physical barrier around the droplets of the dispersed phase. This will thwart the dispersed droplets from meeting and coalescing, preventing instability and phase separation of the emulsion. This is why MFC is considered a stabiliser but not an emulsifier. A schematic demonstration of how the MFC network stabilises dispersed oil droplets is shown in figure 1. below. When oil is mixed into a dispersed MFC network using high shear mixing such as an Ultra-Turrax, the result consists of dispersed oil droplets within the 3D fibre network. Under optimised conditions of concentration and mixing speed, the fibre network holds these oil droplets physically in place avoiding destabilisation of the emulsion. MFC is performing as a stabiliser in this case.
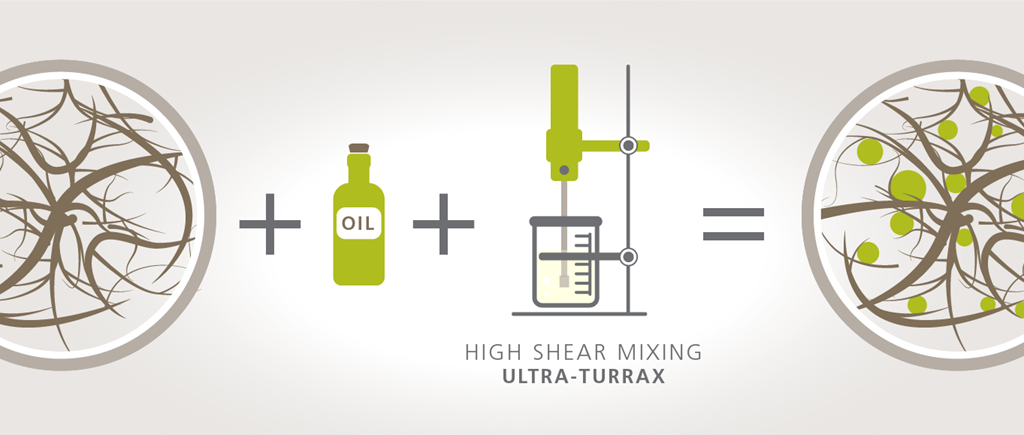
Figure 1: When oil is mixed into a dispersed MFC network using high shear mixing such as an Ultra-Turrax, the result consists of dispersed oil droplets within the 3D fibre network. Under optimised conditions of concentration and mixing speed, the fibre network holds these oil droplets physically in place avoiding destabilisation of the emulsion. MFC is performing as a stabiliser in this case.
As an example, MFC (Exilva® by Borregaard) at a given concentration was dispersed in water and a simple Oil-in-Water emulsion was prepared at room temperature using an Ultra-Turrax mixer at 10 000 rpm for 4 minutes. The emulsion consists of 80% aqueous phase (water and MFC) and 20% oil phase (paraffin oil). The concentration of MFC (the active fibre content) is varied from 0.1 wt% to 1 wt% of the total emulsion to demonstrate the effect of the network and MFC concentration on emulsion stabilisation.
Figure 2. below shows how MFC can stabilise the oil droplets dispersed in water in a simple oil and water mixture. It is becomes clear that increasing the concentration of MFC in the emulsion improves the emulsion stability.
At concentrations above 0.4 wt% active fibre content of MFC, and for this simple oil and water mixture, the MFC 3D network is strong enough to hold the dispersed oil droplets in place and provide total emulsion stability without the need for an emulsifier. The dispersed oil droplets do not sediment or float, flocculate or coalesce, and the emulsions are stable for more than 24 months at room temperature.

Figure 2: Oil-in-water emulsions with Water, MFC (Exilva, Borregaard) and paraffin oil. The concentration of MFC is between 0.1 wt % and 1 wt % active fibre content. MFC is dispersed in the water phase. The emulsion is made of 20% oil phase and 80% water phase. The mixing is carried out at room temperature using an Ultra-Turrax mixer at 10 000 rpm for 4 minutes.
When used at the right concentration of active fibre content and incorporated properly, MFC can function as an emulsion stabiliser. This can lead to the following formulation possibilities:
- Reduce the amount of emulsifier or surfactant needed when using MFC as a stabiliser of your emulsion
- Prepare an emulsifier free formulation using MFC as the stabiliser when possible
In general, in an ideal formulation, one can use MFC to thicken the formulation, make it sprayable with a non-dripping effect and at the same time stabilise the emulsion as well as keep particles and pigments homogeneously dispersed and suspended in the formulation.
Fun fact
How to determine if an emulsion is an O/W or a W/O emulsion?
Many ways exist to do so, but the drop dilution test is very visual and quick. First add a drop of the unknown emulsion in a test tube then add water. Shake the tube.
- If the water mixes with the drop and dilutes it, the unknown emulsion is an oil in water emulsion (O/W). Water is the outer phase/continuous phase of the unknown emulsion.
- If the water does not mix or dilute the drop, the unknown emulsion is a water in oil emulsion (W/O). Oil is the outer phase/continuous phase of the unknown emulsion
