Agglomeration: The Hidden Challenge in Aqueous Anode Production
Consistent battery cell quality starts with mastering mixing and coating. These early steps shape every electrode. One overlooked issue - agglomeration - can disrupt the entire process.
Agglomerates are clusters of poorly dispersed particles. Instead of spreading evenly, they clump together. This causes uneven coatings, irregular pore distribution, and local conductivity variations. These defects grow during drying and calendering, leading to inconsistent layer thickness, weak adhesion, and reduced cell performance.
In large-scale production, such defects multiply. Thousands of cells may be affected, resulting in scrap, rework and, costly downtime. Independent industry analyses confirm that mixing and coating are high-risk steps. Controlling them is essential for stable processes and higher yield.
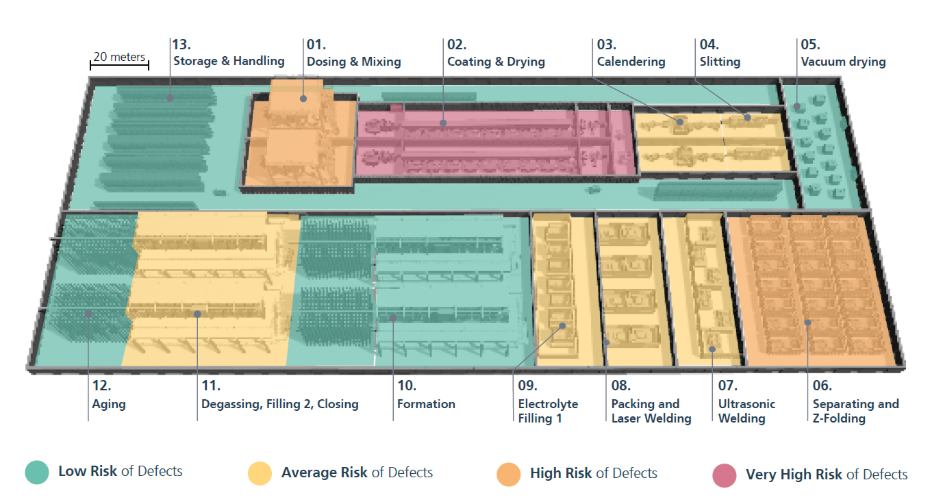
Figure 1. Layout of a typical, modular battery cell production line (7-10 GWh/a) and their risk level for defects. A battery cell factory has multiple modules/lines. Source: www.ffb.fraunhofer.de
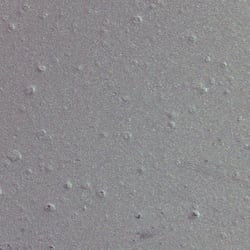
Figure 2. Microscopic image illustrating agglomerated particles within the electrode coating.
Why Agglomeration Occurs
Agglomerates form inside the slurry. A typical anode contains graphite, conductive carbon, binder, thickener, and water. Both graphite and carbon are hydrophobic - they repel water and attract each other. This leads to clumping and gel-like networks that resist flow and hinder coating.
Colloid and interface science explains the forces behind this:
- Polymer bridging: binder molecules such as carboxymethyl cellulose (CMC) or styrene-butadiene rubber (SBR) connect particles.
- Depletion attraction: smaller molecules pull particles together.
- Hydrophobic and van der Waals interactions: strong on carbon surfaces, where water-repelling behaviour and surface attraction cause particles to cluster tightly.
When these forces dominate, slurry viscosity rises. The solid network resists breakdown, even with mixing.
Dispersants Make the Difference
Dispersants solve this challenge. These functional molecules adsorb onto particle surfaces, creating electrostatic or steric repulsion to keep particles apart.
In aqueous systems, dispersants are essential to prevent agglomeration. While CMC and SBR primarily act as binders, lignosulfonates function as powerful dispersants. They stabilise the suspension and influence conductivity, adhesion, and mechanical strength. The right dosage and binder-dispersant interaction determine whether the slurry flows or clumps.
A balanced formulation - optimised dispersant level, mixing energy, binder chemistry, and the right coating settings - delivers a uniform coating and consistent electrode properties.
Mixing: Where Chemistry Meets Process
Even with the right formulation, mixing is crucial. The order of addition, speed, and shear affect particle separation and polymer orientation. Insufficient mixing leaves agglomerates intact.
Excessive shear can degrade polymers or cause re-agglomeration.
Advanced dispersants such as lignosulfonates widen the process window. They maintain dispersion under varying shear conditions, reducing sensitivity to mixing order and energy. This makes large-scale production more robust. Lignosulfonates keep slurry stability across mixer types - from laboratory Thinky mixers to industrial planetary systems. Well-dispersed slurries show predictable rheology, better coating, and improved drying.
Controlling Microstructure
Rheology reveals slurry microstructure. Measuring storage modulus (G’) and loss modulus (G’’) shows how particles interact and when the structure breaks under shear. Lower yield stress and stable viscoelastic behaviour indicate effective dispersion.
This rheological profile links directly to electrode performance. A well-dispersed carbon network improves electron transport and lowers internal resistance - improving energy efficiency and cycle life.
From Challenge to Advantage
Agglomeration is more than a process issue - it is a scientific challenge. Solving it gives you a competitive edge. With the right dispersants and mixing strategy, you reduce defects, improve yield, and unlock better performance from aqueous anodes.
As the industry shifts to sustainable, water-based electrode production, mastering dispersion is essential. It’s the key to stable processes, high-performing batteries, and greener technology.
Ready to take the next step?
.png?width=517&height=128&name=Group%20(2).png)